Engineered adoptive cellular therapy with CD19-targeting chimeric antigen receptor T-cells (CART) has revolutionized the treatment landscape for patients with relapsed/refractory (R/R) large B cell lymphoma (LBCL); however, failure rates remain high. Thus, improved molecular understanding of patient and treatment characteristics linked to response are urgently needed to define the basis for achieving sustained remission.
Single cell technologies have the power to evaluate heterogenous populations and provide unprecedented ability to track T cell clonal kinetics. Here we focused on patients with R/R LBCL treated with axicabtagene ciloleucel (axi-cel), from whom we collected serial peripheral blood mononuclear cell (PBMC) samples from Day -30 to +28 relative to CAR-T infusion, as well as bag washings from infusion products (IPs). We evaluated 308,357 single cell transcriptomes (10x Genomics) from serial PBMCs and IPs from 29 patients (16 responders [R], 13 non-responders [NR], R defined as complete [CR] or partial response [PR] at 1 year, NR defined as progressive disease [PD] by 1 year), of which 220,653 originated from 19 patients in a previously reported cohort generated from our center (Haradhvala et al., Nat Med, 2022) and 87,704 newly generated from 10 of 50 patients (29 R, 21 NR) from an extension cohort on whom flow cytometry and mass cytometry (CyTOF) were also generated. With this expanded power, we systematically evaluated baseline and immediate post-treatment samples for features associated with durable response.
While we observed no quantitative differences in post-infusion circulating immune subsets in R versus NR, scRNA-seq analysis yielded two notable findings at baseline (Day -30). First, the proportion of B cells (mostly naïve B cells) was increased in R (n=12) compared to NR (n=7) (p=0.012). Protein expression by flow cytometry in the full extension cohort (of whom 20 [13 R, 7 NR] had available PBMC data at baseline) revealed a trend towards increased B cells in R (p=0.16), dominated by four exceptional outliers in whom B cells comprised 2-30% of total PBMCs, all of whom were CR. Resegregating these 20 patients to 11 CR vs 9 non-CR confirmed higher frequency of circulating B cells (median CR 0.18% vs non-CR 0.011%, p=0.0057). Patients with B cells had longer time from anti-CD20 therapy (60, 41, 22, and 10 months) whereas patients without B cells had median 1 month since last anti-CD20 therapy. Second, lymphocyte to monocyte ratio [ALC/AMC] by scRNA-seq trended higher in R (n=12) compared to NR (n=7) (median 1.36 vs 1.02, p=0.22). This was confirmed in the larger flow cytometry cohort (median 2.38 vs 0.69, p=0.0013) and supported by both increased percent of total lymphocytes in R (n=13) vs NR (n=7) (median 31% vs 9.4%, p=0.011) as well as decreased percent of monocytes in R vs NR (median 68% vs 91%, p=0.019). As validation, we detected an elevated ALC/AMC upon evaluation of our entire cohort of patients treated with commercial axi-cel at DFCI from 2019-2022 (n=157 R, 123 NR) based on complete blood count and differential on Day -30 (median 1.31 for R vs 0.97 for NR, p=0.0034).
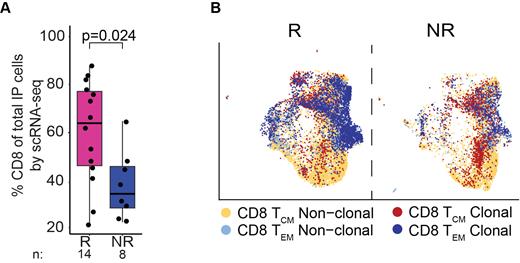
Since we detected evidence of immunologic differences at baseline between R and NR, we asked whether differences in IPs contributed to therapy outcome. By scRNA-seq, IP cells demonstrated increased proportion of CD8 T cells in R (62% vs 32%, p=0.024, Fig 1A) with a trend toward higher proportion of a T effector memory (T EM) expression profile (median 45% vs 21%, p=0.079). By scTCR-seq analysis, we observed greater clonal expansion of CD8 T EM cells in R vs NR, whereas non-clonally expanded cells tended towards a T CM profile (median ratio of clonal TEM to clonal TCM 2.61 for R vs 0.71 for NR, p=0.042, Fig 1B). Differential gene expression of clonally expanded vs nonclonally expanded CD8 T cells revealed increased expression of T cell activation genes ( GZMH, IFNG, TNF, KLRD1, B3GAT1, HLA-class II genes) among clonally expanded cells. These data implicate clonal expansion and T cell activation at infusion as key indicators of CAR-T response.
Our results suggest that baseline patient immunologic state affects likelihood of response to CART through modulation of the T cell apheresis product composition and promoting a more favorable circulating immune compartment prior to therapy. These features, detectable by highly accessible clinical measures, could be leveraged for improved patient selection and enhanced CAR-T manufacturing.
Disclosures
Houot:Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi: Consultancy; Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, F. Hoffmann-La Roche Ltd: Honoraria. Gohil:UCLB: Patents & Royalties; Novalgen: Consultancy; Freeline Therapeutics: Consultancy; Janssen: Speakers Bureau; Abbvie: Honoraria, Other: Travel; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Speakers Bureau. Miles:Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company. Mattie:Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company. Livak:Standard BioTools Inc: Current equity holder in publicly-traded company; MBQ Pharma Inc: Consultancy. Ritz:Garuda Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; LifeVault Bio: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; TScan Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Clade Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Smart Immune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Research Funding; Oncternal: Research Funding; Equillium: Research Funding; Avrobio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Akron Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees. Rodig:Immunitas Therapeutics: Membership on an entity's Board of Directors or advisory committees; KITE/Gilead: Research Funding; Bristol Myers Squibb: Research Funding. Armand:Xencor: Consultancy; IGM: Research Funding; Adaptive Biotechnologies: Research Funding; ATB Therapeutics: Consultancy; Foresight Diagnostics: Consultancy; Genentech/Roche: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Tessa Therapeutics: Consultancy; Regeneron: Consultancy; Enterome: Consultancy; Affimed Therapeutics: Research Funding; Kite - a Gilead company: Research Funding; MSD: Consultancy, Research Funding; GenMab: Consultancy; ADC Therapeutics: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding; Merck: Consultancy, Honoraria, Research Funding. Wu:BioNTech Inc: Current equity holder in publicly-traded company; Pharmacyclics: Research Funding. Jacobson:Kite, a Gilead company: Consultancy, Research Funding; Novartis: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Abbvie: Consultancy; ADC Therapeutics: Consultancy; AstraZeneca: Consultancy; Abintus Bio: Consultancy; Caribou Bio: Consultancy; Instil Bio: Consultancy; ImmPACT Bio: Consultancy; Daiichi-Sankyo: Consultancy; Ipsen: Consultancy; Morphosys: Consultancy; Synthekine: Consultancy; Pfizer: Research Funding; Miltenyi Biotec: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal